Click the link to read the article on the Big Pivots website (Allen Best):
April 18, 2024
Bill moving through Colorado Capitol that would allow Xcel Energy and Tri-State G&T to keep water rights for 20 years after last coal plant closes
Colorado’s Yampa River Valley has five coal-burning units that will cease operations from 2025 to 2030. Two are at Hayden and three are at Craig. All require water for cooling.
What will become of that water once the coal plants close?
SB24-197, a bill that is rapidly moving through the Colorado Legislature, would allow Xcel Energy and Tri-State Power and Generation to hold onto their water rights, even if they are not using them, until 2050. That is a precedent-setting exception to Colorado’s famous use-it-or-lose-it provision in water law.
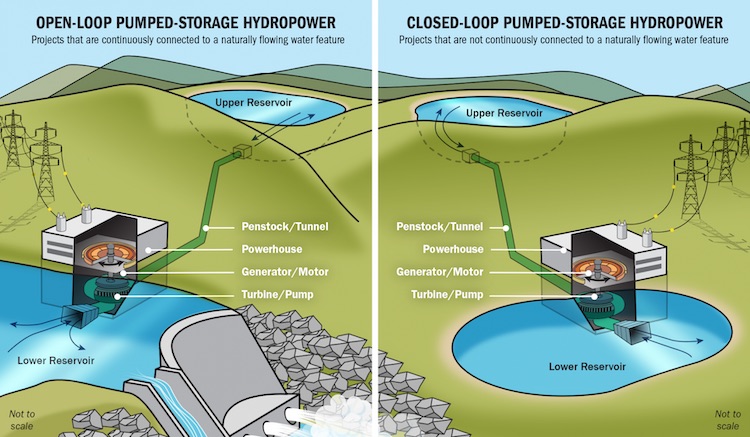
The utilities say they may very likely need the water once they figure out how they will replace the coal generation. Neither utility has announced specific plans, but in response to a question at the bill’s first hearing in a Senate committee last week, Xcel Energy’s Richard Belt identified pumped-storage hydro and hydrogen as leading candidates. The federal government has devoted considerable funding and support for development of both technologies, he said.
“Those are the two leaders,” said Belt. “There aren’t many on the horizon that would fill the niche in that decree.”
Both technologies would provide storage. Xcel and other utilities are on their way to having massive amounts of cheap renewable energy. Still to be solved is how to ensure reliability when winds quiet for long periods. And the sun, of course, always goes down.
Storage will be essential and perhaps some kind of baseload generation. Xcel’s current plans call for an increase in natural gas capacity to ensure reliability even if the natural gas plants are used only infrequently, say 1% or 2% of the time. Xcel Energy is also adding literally tons of four-hour lithium-ion battery storage.
The company’s biggest storage device is still its oldest, the 324-megawatt Cabin Creek pumped storage unit. Water from the upper reservoir is released to generate electricity when it is needed most, then pumped back uphill when power is relatively plentiful.
A developer has secured rights from landowners at a site between Hayden and Craig. See story. Another pumped-storage hydro possibility has been identified in the area between Penrose and Colorado Springs.
Hydrogen has less of a track record, at least in Colorado. However, it is part of Colorado’s all-of-the-above approach. See story. Hydrogen can be created from natural gas, but to meet Colorado’s needs it must be created from water. It would then be stored. Like pumped-storage hydro, it would be created when renewables are producing excess electricity, and the hydrogen could then be tapped to create electricity when needed most. That electrical generation would also use water for cooling, Belt said.
The bill, said Belt, proposes to allow Xcel the time for the economic and feasibility details of these emerging technologies to be resolved “instead of forcing a near-term decision driven by the processes of current water law.”
Normally, utilities would be required to demonstrate purpose of water, which can take several years, or risk abandonment. Because they will not have to, some see this as allowing the utilities to speculate. The utilities insist that it’s too soon to know exactly what their future water needs will be. But in addition to owning land in the Yampa Valley and water, they have expensive transmission linked to the rest of Colorado.
State Sen. Cleave Simpson, a Republican from Alamosa — and a former lignite coal-mining engineer, made note of that infrastructure on the floor of the Senate on Monday morning when he spoke in favor.
The bill will allow the utilities to hold onto the water in Western Colorado “so the region can have a true just transition and so hopefully it can continue to be an energy producing
region using existing infrastructure.” Upon advice of the Colorado Attorney General’s Office, the bill was amended by the Senate to specify that the water must remain in the Yampa River Basin.
Since Colorado adopted carbon reduction targets in 2019, there have been questions about what might happen to the water in the Yampa Valley. It’s not a huge amount of water, but it can matter in a basin that since 2018 has had several calls on the river after having none for the previous 150 years.
The issue was hashed out by the legislatively-created Drought Task Force in 2023. The task force called attention to the idea of allowing utilities to preserve their water rights until 2050, but the idea failed to get a full endorsement.
Sen. Dylan Roberts, a prime sponsor, explained at the Senate Agriculture and Natural Resources Committee meeting that additional work in recent months has produced legislation that has ended objections. Indeed, Western Resource Advocates supported the full bill, as did others.
Jackie Brown, who represented Tri-State on the task force, told the Senate committee members that the measures in SB24-197 “provide Tri-State certainty that our water resources remain intact and available for future dispatchable carbon-free generation as needed and is projected in our electric resource plan. While we continue our planning process, keeping this utility water in the Yampa River helps all water users, creating a win-win situation.”
The Glenwood Springs-based Colorado River District in 2021 conducted a study of what happens to water when released from the Elkhead Reservoir, which is located near Hayden. The study found that 14% of the water was picked up by irrigators, 10% was lost to transit – and the rest of it flowed downstream. That suggests what will become of this water while it is not used.
Downstream lie segments of the Yampa where endangered fish species live. Those stretches have become nearly non-existent during the hot and dry summers of recent years.
Routt County Commissioner Tim Corrigan said his county supports the bill. He said hebelieved that Moffat County did also. He emphasized that the solution will help the environment as well as other users. The energy transition in northwest Colorado, he said “will take place over a very long time.”
The bill also has provisions applicable across Colorado. It allows the owner of a decreed storage water right to loan water to the Colorado Water Conservation Board for a reach of river for which the board does not hold a decreed instream flow water right. It also requires the CWCB to establish an agricultural water protection program in each of the state’s water divisions.
Simpson, on the Senate floor, also explained that the bill would create what he called a much-needed program, crafting a pathway to loan water from water storage for a reservoir to benefit an instream flow program “without going through the whole CWCB process with getting an adjudicated flow.”